RESEARCH
From Molecular Genetics to Translational Therapeutics
Molecular and Function characterization of Novel Intellectual disability associated genes
Neurological conditions account for over 6% of the global disease burden. There are more than 600 neurological disorders and cognitive dysfunction, also referred to as intellectual disability (ID), occupies a prominent position in this list. Cognitive dysfunction arises from the failure of neuronal cells to organize into a complex network and remodel this network in response to learning and experience. It is manifested by deficits in adaptive behaviors in everyday social and practical skills. Due to its high prevalence and the lifetime cost of care per individual in the range of $1-2 million in the United States (CDC), it presents a significant health burden. An emerging understanding of ID posits a seed genetic cause that escalates across the scales and networks of the nervous system into a systems-level perturbation of information processing in multiple brain areas, leading to pathologies of cognition. Etiopathogenesis can be found in one protein often implicated in various cellular processes and developmental stages. During the interdependent processes of brain circuit development, these perturbations cause cascades of dysfunction that ripple across the molecular and cellular scales and scales of neuronal connectivity, from synaptic nanostructure to transmission to mesoscale neuroanatomical connectivity. Monogenic ID, therefore, presents a rare opportunity to appreciate the tiers of causality in diseases of cognition and those of neurotypical brain development when investigated across scales and developmental time: Dr. Saima Riazuddin and her collaborators, including Drs. Thomas Blanpied and Alex Poulopoulos at UMB have team synergy to investigate ID from its genetic to its circuit miswiring etiologies by interrogating distinct cell types (cellular scale), the composition and distribution of molecular complexes (nanoscale), and the connectivity networks they develop across the brain (mesoscale), investigated in variety systems and developmental times. We use this integrated approach as a proof-of-principal pipeline for investigating nervous system diseases stemming from monogenic causes that lead to complex cognitive impairment.
SLC8A3, NAV3, KLHL14 and LLGL2 are examples of novel ID associated genes we identified and currently functionally characterizing in the Riazuddin lab
Identification of Genes Causing Syndromic and Nonsyndromic Hearing Impairment
Genetics of Hearing Disorders (Go-HEAR) research program in Dr. Saima Riazuddin’s lab is designed to identify and characterize proteins essential to human inner ear development, neurosensory function and long-term maintenance. Hearing loss (HL) is a highly variable phenotype that affects >360 million people worldwide. Genetics and functional studies of the HL-associated proteins have helped to elucidate the molecular components of inner ear tissue, but the molecular identities of many essential components of inner ear neurosensory cells are still unknown. Transcriptome analysis revealed ~18,199 genes expressed in the organ of Corti’s hair and supporting cells, underscoring the complexity of inner ear development and function. The long-term goals of the Go-Hear research program are to elucidate molecular networks essential for the development, maintenance, and neurosensory function of human inner ear, to help decipher mediating mechanisms and develop new therapeutic agents for treatment and prevention of HL. Go-HEAR research program employs advanced techniques to identify new genes, variants, and characterize their roles in pathways that contribute to NSRHL, in a large, unique, advantageous cohort of human HL families. Discovering a HL-causing human gene rules out functional redundancy and informs about its essential role in hearing.
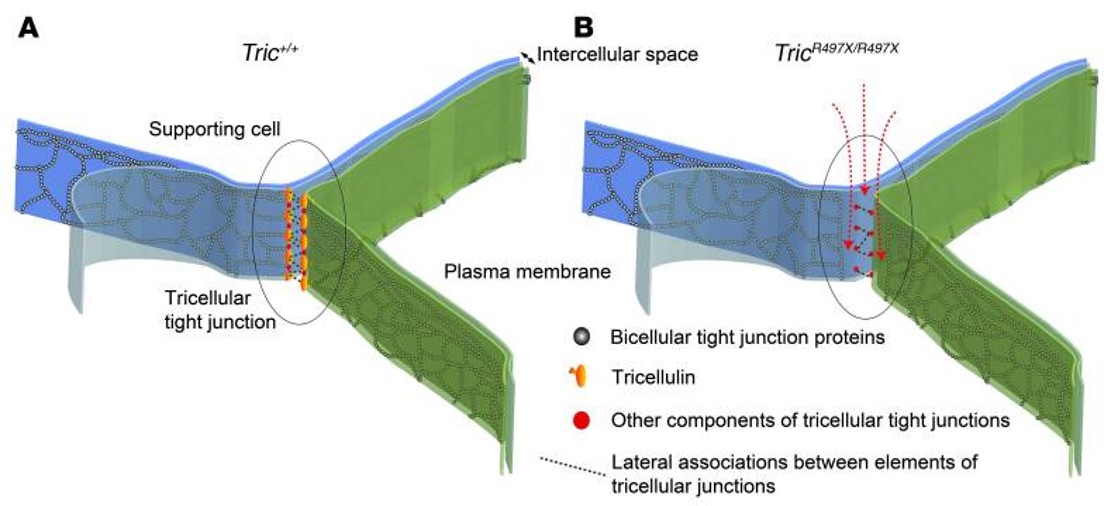
Dr. Riazuddin has built a useful resource of families with hearing disorders in Pakistan and Africa, enabling her team to map new HL loci, detect novel variants in known genes, and identify novel genes. This resource of families has led to the mapping of 13 novel HL loci, 11 of which were non-syndromic. Her team also identified or contributed in the identification of 12 novel HL genes using these families. They have also identified novel variants in various known HL genes in these families, including MYO15A, SLC26A4, TRIOBP, FGF3, CDH23, TRIC, and an X-linked gene. In 2019, in a multi-center collaboration, her team published the most comprehensive genetics analysis and variants of known deafness genes found in 321 Pakistani families. In this study, Sanger and exome sequencing revealed 163 DNA variants, including 70 novel alleles of 41 known HL genes. Her team also characterized the function of several HL-associated proteins in the inner ear using mouse models, including Tricellulin, MAP3K1, CIB2, MSRA, MSRB1, MSRB2, MSRB3, Occludin, etc.
Role of CIB Proteins in the Inner Ear Structure and Function
Hearing loss (HL) is an etiologically diverse condition that can occur at any age and severity level, affecting 1 in 500 infants and more than 360 million people globally. In numerous ethnicities, Dr. Ahmed and his team have previously identified pathogenic variants in the CIB2 gene encoding Calcium and Integrin-Binding protein 2 (CIB2) as the etiology of HL. In collaboration with Dr. Gregory Frolenkov at the University of Kentucky, his team discovered that CIB2 is expressed in the mouse hair cell stereocilia and binds to the TMC1 and TMC2 components of the hair cell mechanoelectrical transduction (MET) complex and that deafness causing CIB2 mutations disrupt these interactions and concluded that CIB2 is essential for the MET function. His team has generated two mouse models carrying the human deafness-related Cib2 variants (Cib2F91S and Cib2R186W knock-ins) and characterized them together with a mouse line lacking CIB2 (Cib2ko). Both Cib2Fand Cib2ko mouse strains are deaf and lack typical MET responses in the auditory hair cells despite the existence of tip links that are ordinarily responsible for gating the MET channels. In contrast, the p.R186W mutation does not disrupt the interaction between CIB2 and TMC1/2 and MET currents in mutant mice are diminished but still detectable. It is particularly intriguing that CIB2’s participation in the MET machinery may be responsible for at least some of the several well-known effects of Ca2+ on hair cell MET function. Currently, his team, in collaboration with Dr. Frolenkov, is investigating the precise function of CIB2 in MET. Their working hypothesis is that CIB2 is a calcium-dependent element that regulates the sensitivity of the MET channels and force transmission to these channels in the mammalian auditory hair cells.
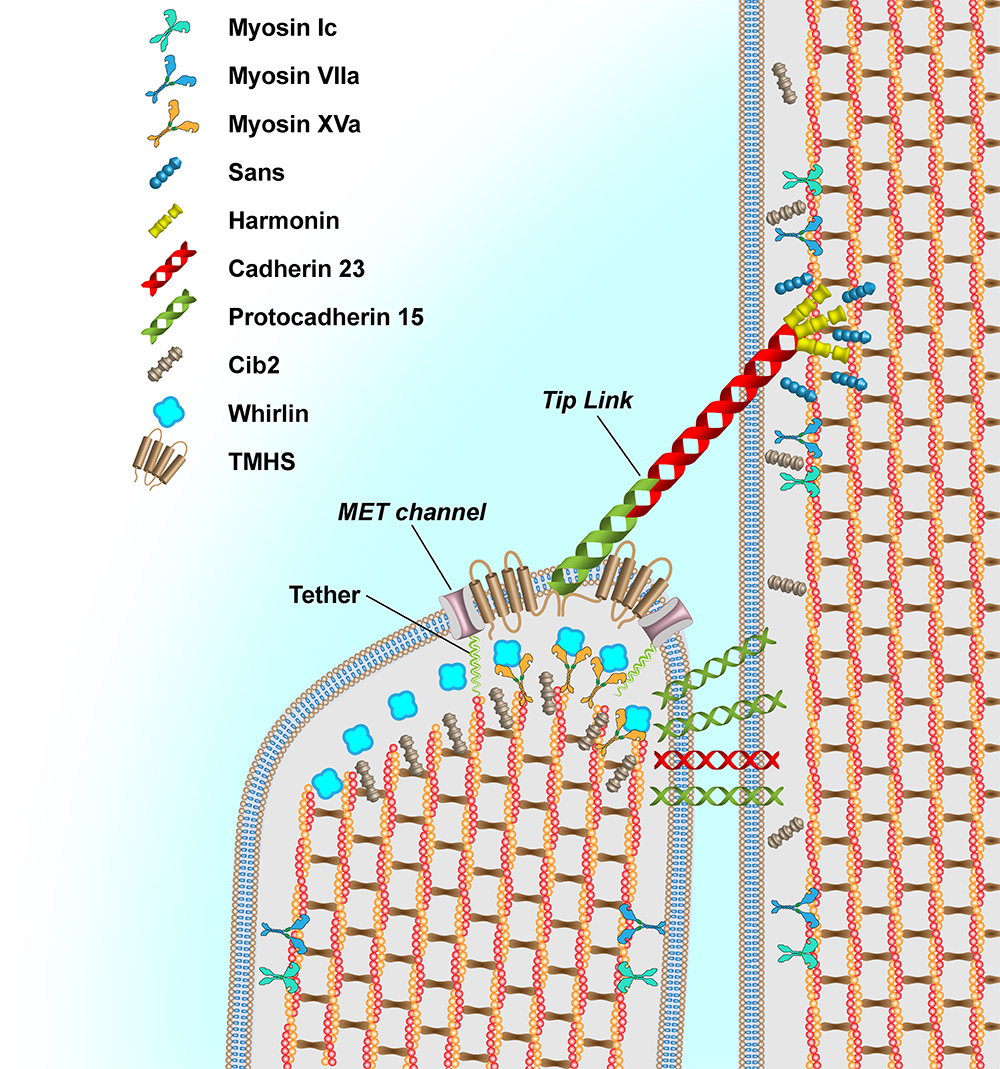
Their findings further determined that CIB2 deficiency causes an overgrowth of transducing shorter-row stereocilia in the hair bundle without altering the non-transducing tallest-row stereocilia. This observation cannot be explained solely by the loss of MET since blockage of the MET channels causes an opposite effect, the retraction of transducing stereocilia. Hence, CIB2 must have some role in stereocilia growth, unrelated to MET. Currently, they are investigating the involvement of CIB2 in the molecular networks implicated in the development and patterning of auditory stereocilia bundles. Besides CIB2, Dr. Ahmed’s team is also investigating the role of CIB3 in the auditory and vestibular hair cells.
Role of CIB2 in the regulation of Autophagy
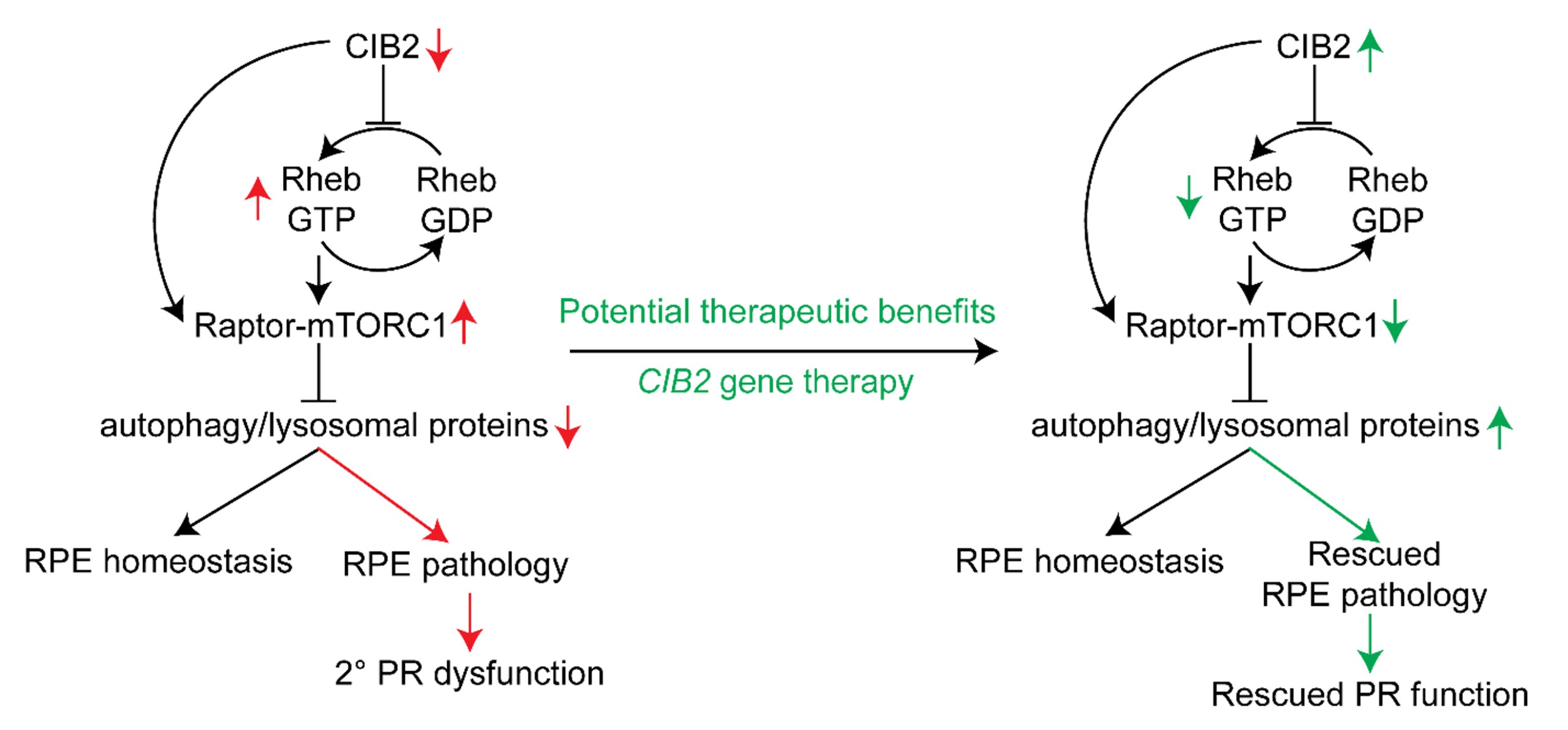
Autophagy is the basic cellular process of eliminating damaged/old proteins/organelles and cellular debris to maintain function of all cells. Deficits in autophagy have been associated with age-related pathology in mice and humans. Previously, we reported that calcium and integrin-binding protein 2 (CIB2) regulates autophagy in the retinal pigment epithelium (RPE) via mechanistic target of rapamycin complex 1 (mTORC1) signaling. Mice lacking CIB2, specifically within RPE, have age related pathologies, including sub-RPE deposits, vacuolization, lipid accumulation, and impaired visual function. CIB2 depletion also caused reduced autophagic clearance, and increased mTORC1 signaling – a negative regulator of autophagy. RPE cells cultured from patients suffering with age-related macular degeneration (AMD) showed elevated numbers of autophagosomes, lipid droplets, aberrantly large lysosomes, and higher starvation-induced SQSTM1/p62 levels. Consistent with these observations, we found aberrant autophagy in RPE/choroid samples from dry AMD patients. These data, together, reconcile CIB2 levels, mTORC1 activity, autophagy, and their age-associated impact on the RPE maintenance and function in mice and humans. Hence CIB2 potentially stands at the interaction of several diseases, with dry AMD – for which there are limited to no treatment options. To test CIB2 gene delivery approach, we developed an AAV (Adeno-Associated Virus) vector to deliver a functional hCIB2 gene in the mouse retina. Mice injected with hCIB2 demonstrated a significant improvement in photopic visual acuity, improvement in several AMD-like phenotypes including reduction of sub-RPE deposits, and drusen proteins and lipids. We also found marked improvement in autophagy and a significant downregulation of the mTORC1 signaling pathway. The data suggested that delivery of hCIB2 has the potential to effectively reverse the pathology of RPE drusen and improve autophagy by downregulating mTORC1, which is typically associated with dry-AMD. These findings support the notion that AAV-mediated CIB2 delivery could be a promising therapy for individuals suffering from dry-AMD.
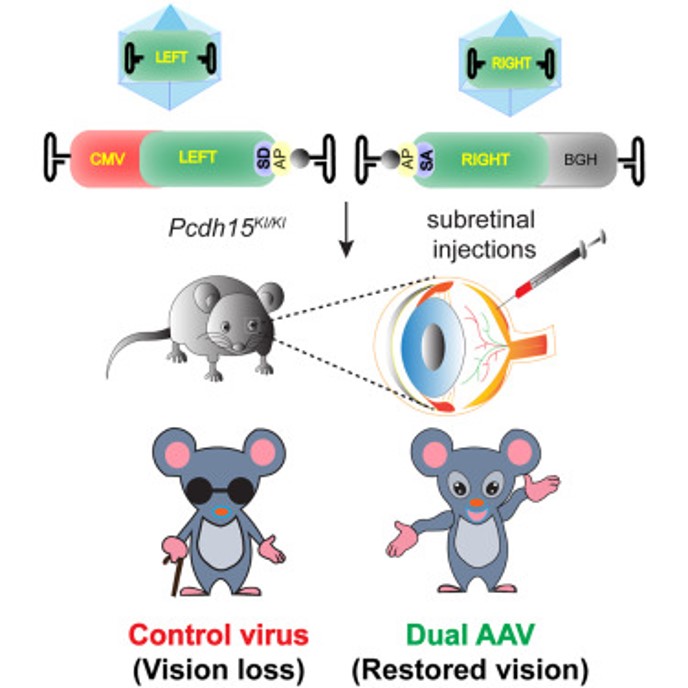
Therapeutic Approaches to Restore Vision in the Preclinical Animal models
Mutations in the PCDH15 gene, encoding protocadherin-15, are among the leading causes of Usher syndrome type 1 (USH1F), accounting for up to 12% of USH1 cases worldwide. A founder truncating variant of PCDH15 has a ∼2% carrier frequency in Ashkenazi Jews, accounting for nearly 60% of their USH1 cases. Although cochlear implants can restore hearing perception in USH1 patients, there are no effective treatments for vision loss due to retinitis pigmentosa. We established a founder allele specific Pcdh15 knockin mouse model as a platform to ascertain therapeutic strategies. Using a dual-vector approach to circumvent the size limitation of adeno-associated virus, we observed robust expression of exogenous PCDH15 in the retinae of Pcdh15KI mice, sustained recovery of electroretinogram amplitudes and key retinoid oxime, substantially improved light-dependent translocation of phototransduction proteins, and enhanced levels of retinal pigment epithelium-derived enzymes. Thus, our data raise hope and pave the way for future gene therapy trials in USH1F subjects.
Currently, we are testing multiple translational approaches, such as CRISPR-based editing, read-through compound delivery, and AAV-EVs, to treat blindness in Pcdh15KI mice and Pcdh15 humanized mice.