Centrioles organize centrosomes which function in spindle formation, cell cycle progression, and directing the organisation of the interphase cell. They also direct formation of cilia, which are critical for multiple signaling processes and motility.
For over a century, supernumerary centrosomes have been known to be found in cancer cells. Moreover, defective centrosomes prevent the correct development of the cortex of the brain in the microcephaly disease family and defective centrioles and cilia result in a large complex of inherited diseases collectively known as the ciliopathies.
We wish to understand the processes whereby the centriole duplicates to organize centrosomes and cilia.
The centriole duplication cycle
Genetic studies in C. elegans first identified the canonical duplication pathway: SPD2 targets ZYG1 kinase to centrioles upstream of SAS5 and SAS6 that in turn act before SAS4. Studies from our lab and from Erich Nigg’s showed that the related Plk4 kinase drives canonical centriole duplication and de novo formation in human and Drosophila cells. Subsequently, we and others showed that Plk4 levels are regulated through autophosphorylation that enables binding to the F-box protein of SCF to mediate its destruction. We identified its other main partner to be Asterless (Asl); Asl targets Plk4 to centrioles in Drosophila, in human cells Asl (Cep152) shares that task with human Spd2 (Cep192). (Dzhindzhev et al. Nature 2010)
We then showed that Drosophila Plk4 phosphorylates Ana2, the fly counterpart of SAS5, to enable it to recruit its Sas6 partner in procentriole formation after centriole disengagement in telophase; this mechanism is conserved in human cells.
We now aim to determine how phosphorylation of Ana2 and the Polo-like kinase’s other substrates influence their interactions with both characterised and as yet poorly characterized core components of the centriole and how this ensures procentriole formation at a single site. (Dzhindzhev et al. Curr Biol. 2014; Dzhindzhev et al. Open Biol. 2017)
The microtubules of the procentriole are fully elongated by the time a cell is ready to enter into mitosis. It is now a fully fledged daughter centriole, but it is still unable to duplicate or to organize pericentriolar material (PCM). The daughter centriole acquires these properties during passage through mitosis when it is converted into a centrosome.
We recently described the hierarchy of assembly of a complex that extends radially from the core of the centriole to its periphery enabling this conversion process in cultured fly cells. Key to this is the protein Ana1 which forms a complex with the Sas6-associated Cep135 to load them onto the centriole. Asl can then be recruited onto Ana1 and can then recruit both Plk4 and PCM. The main features of this process are conserved in human cells. (Fu et al. Nat Cell Biol. 2016)
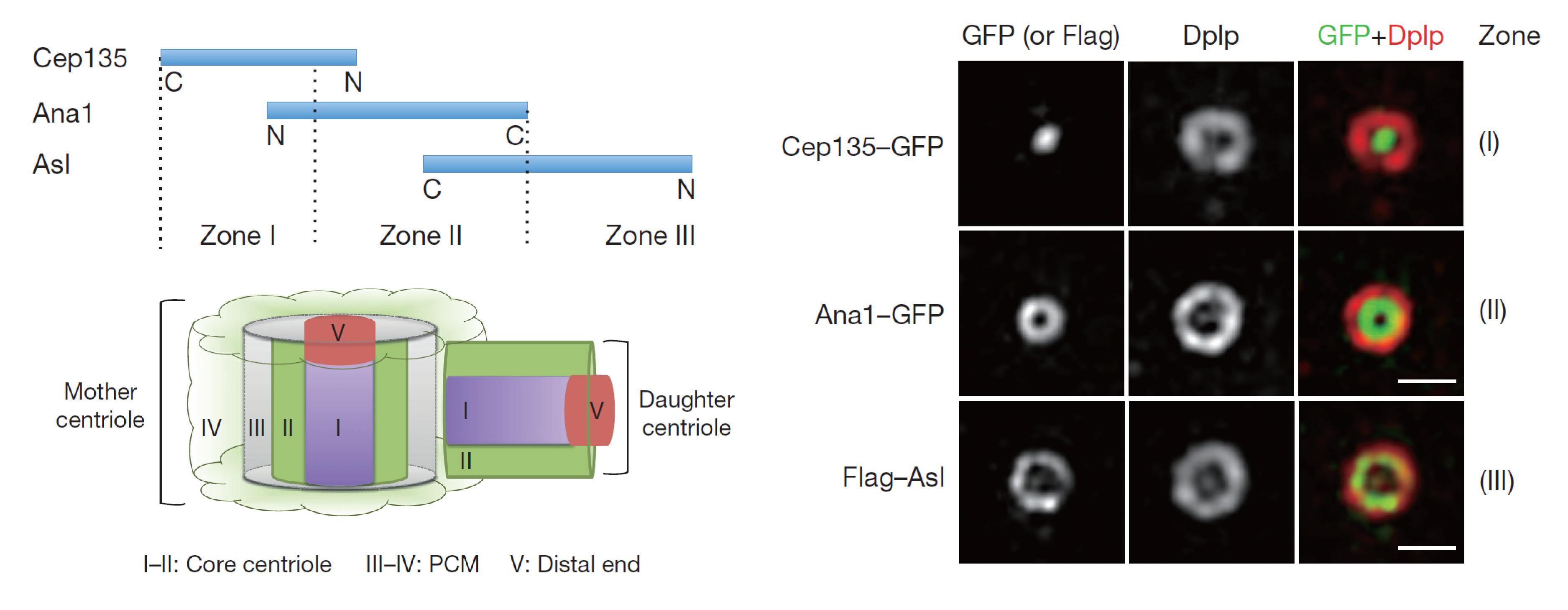
Reference: Fu J, Lipinszki Z, Rangone H, Min M, Mykura C, Chao-Chu J, Schneider S, Dzhindzhev NS, Gottardo M, Riparbelli MG, Callaini G, Glover DM. Conserved molecular interactions in centriole-to-centrosome conversion. Nat Cell Biol. 2016; 18 (1): 87-99. DOI: 10.1038/ncb3274.
Centriole to Centrosome Conversion
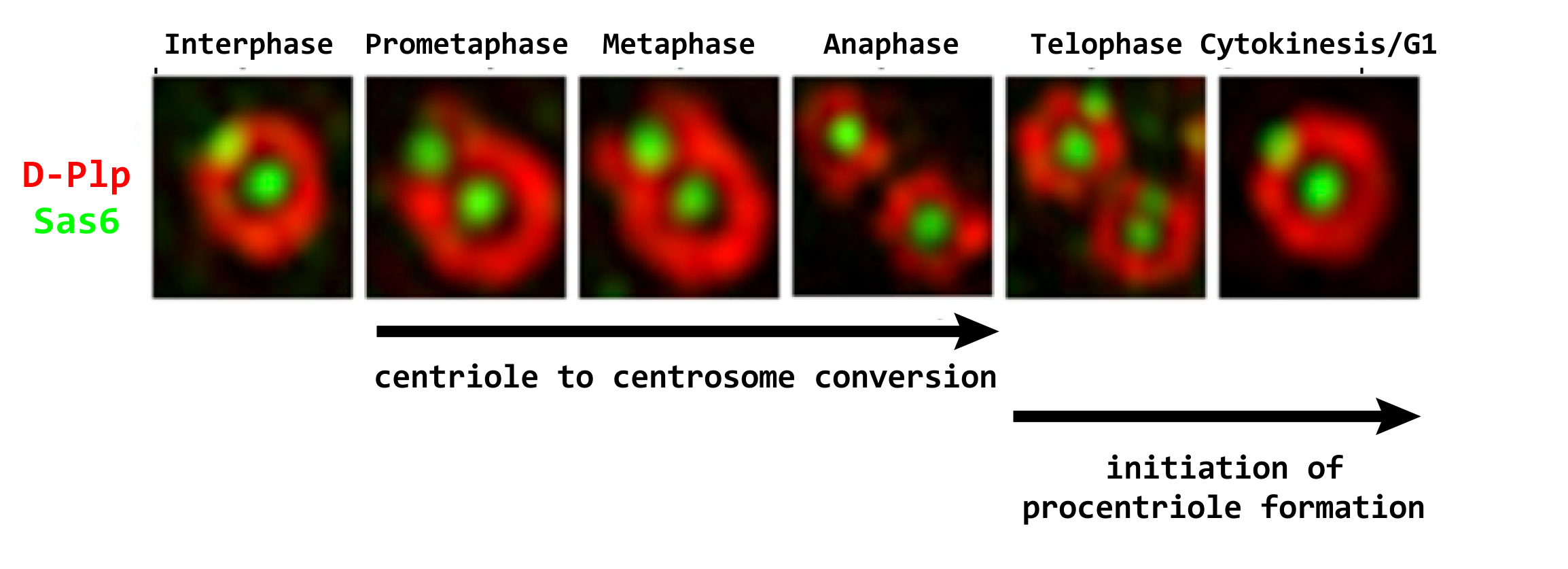
Once the 9-fold symmetrical structure is established, the daughter centriole grows to its full length, predominantly in G2 phase. Once achieved the daughter is converted during mitosis into a structure able to nucleate cytoplasmic microtubules, the centrosome. We showed that centriole-to-centrosome conversion requires that the inner-zone centriole protein Cep135 is recruited together with Ana1 to the daughter centriole in the final stages of its biogenesis immediately before mitotic entry and this is followed shortly after by Asl/Cep152 recruitment. More recently we showed that in addition to Cep135, the Rcd4/PPP1R35 molecule is also required for Ana1 recruitment in somatic centrioles but curiously not spermatocyte giant centrioles. We now wish to determine the functional inter-relationships of these proteins in centriole to centrosome conversion and also centriole elongation. (Fu et al. Nat Cell Biol. 2016)
The centriole and the golgi
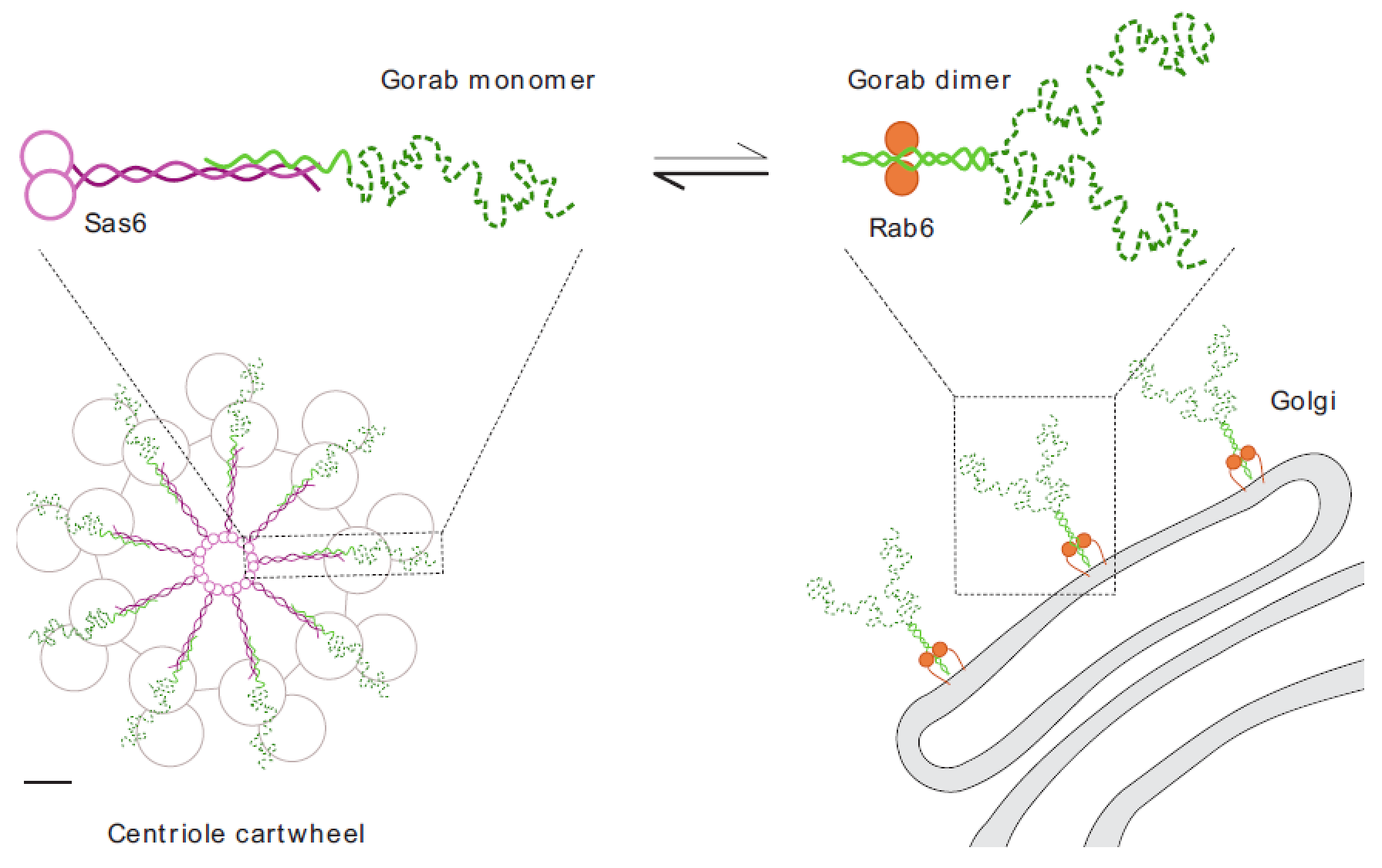
We recently described an unexpected interaction between Sas6 and a trans-Golgi associated protein, Gorab, that is essential to establish 9-fold symmetry of the centriole and which is necessary for both centriole duplication and basal body formation in Drosophila.
The human counterpart of Gorab, which is also associated with the trans-golgi, is mutated in the wrinkly skin disease, gerodermia osteodysplastica. By copying a missense mutation from gerodermia patients we created mutant Drosophila Gorab unable to localise to trans-golgi but still able to rescue centriole and cilia defects of Gorab null flies. We also found that expression of C-terminally tagged Gorab disrupts golgi functions in cytokinesis of male meiosis, a dominant phenotype that can be overcome by a second mutation preventing golgi targeting. Thus, centriole and golgi functions of Gorab are separable.
Reference: Kovacs L, Chao-Chu J, Schneider S, Gottardo M, Tzolovsky G, Dzhindzhev NS, Riparbelli MG, Callaini G, Glover DM. Gorab is a Golgi protein required for structure and duplication of Drosophila centrioles. Nat Genet. 2018; 50 (7): 1021-1031. DOI: 10.1038/s41588-018-0149-1.
We recently used hydrogen-deuterium exchange mass spectrometry to define Gorab’s interacting surfaces that mediate its subcellular localization. A core stabilization sequence within Gorab’s C-terminal coiled-coil domain enables homodimerization, binding to Rab6, and thereby trans-golgi localization. However, part of the Gorab monomer’s coiled-coil domain undergoes a high affinity antiparallel interaction with a segment of the parallel coiled-coil dimer of Sas6 to form a stable heterotrimeric complex. Mutation of a single leucine residue in Sas6’s Gorab-binding domain generates a Sas6 variant with a sixteenfold reduced binding affinity for Gorab that cannot support centriole duplication. Thus, Gorab dimers at the golgi exist in equilibrium with Sas6-associated monomers at the centriole to balance Gorab’s dual role. (Kovacs et al. Nat Genet. 2018)
Ciliary Function in the Developing Peripheral Nervous System and Testes
To address the puzzle of the partially overlapping function of centriole proteins throughout development, we study two Drosophila tissues in which dividing cells use centrioles to organise centrosomes at their spindle poles and differentiating cells develop basal bodies that template axonemal structures. These are the leg imaginal disc, in which ciliated neurosensory cells are formed, and the testes, which produces flagellated sperm. In the neurosensory organs, cilia with doublet microtubules are generated from basal bodies having similar morphology to the centrioles of their precursor cells. In the male germ line, the centriole undergoes a dramatic transition between the gonial mitoses and the primary spermatocyte in which its triplet microtubules elongate by almost 30-fold. This growth phase imposes a different set of requirements upon molecules used in phases 2 and 3 of conventional duplication. Moreover, the resulting pair of primary cilia differ from other cilia in being able to re-acquire the characteristics of centrosomes on meiotic entry. Finally, at the completion of meiosis, the giant centrioles template formation of sperm axonemes, whose morphology differ dramatically from the primary cilia of spermatocytes. Our aim is to determine how the same set of molecules are used to generate these differing centrioles/basal bodies in these two different tissues. (Panda et al. J Cell Biol. 2020)
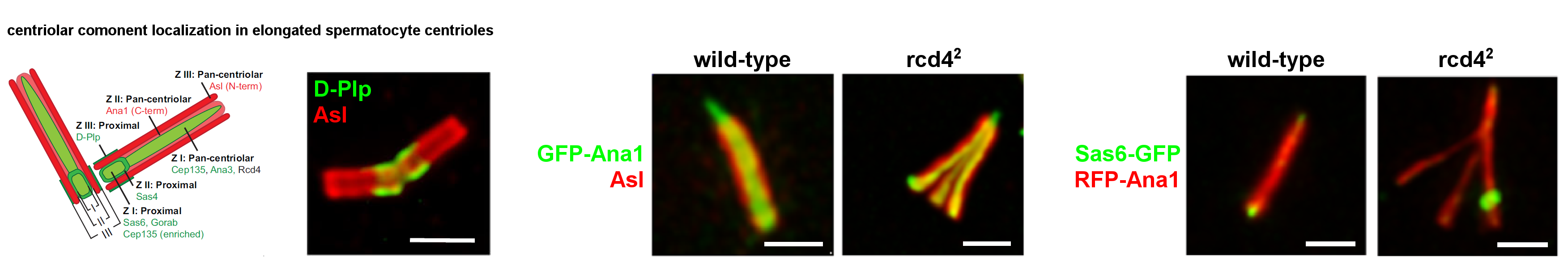
As daughter centrioles assemble during G2, they recruit the conserved Ana3/RTTN protein followed by its partner Rcd4/PPP1R35. Together, this contributes to the subsequent recruitment of Ana1/CEP295, required for the centriole’s conversion to a centrosome. In addition, Rcd4/PPP1R35 is required to maintain 9-fold centriole symmetry in centrioles of the Drosophila male germline. In the absence of Rcd4 protein, microtubule triplets become dispersed into a reduced number of doublet or singlet microtubules. The resulting skinny centrioles of rcd4-null mutant spermatocytes elongate normally and localize centriolar components at the correct positions along the length of the centriole. The rcd4 mutant spermatocytes also have centrioles of normal dimensions that splay at their proximal ends when induced to elongate by elevated levels of Ana1. Strikingly, skinny and splayed spermatid centrioles can still recruit a proximal centriole-like (PCL) structure that represents the initiation of centriole duplication in developing sperm. Thus, the phenotype of the rcd4 mutant tells us that stable 9-fold symmetry of microtubule triplets is not essential for centriole growth, correct longitudinal association of centriole components, and aspects of centriole duplication. We are currently working to understand how the Rcd4::Ana3 complex regulates the stability of centriole microtubules. (Panda et al. Nat Commun. 2024)
Supernumerary centrosomes in the mouse
To address the long-standing question of what might multiple centrosomes be doing in tumour cells, we decided to make a mouse in which we could induce Plk4 expression and hence centrosome overduplication. We found that Plk4 over-expression leads to hyper-proliferation of the skin exacerbated by loss of p53; it also advances tumour formation in p53 null mice.
Mice overexpressing Plk4 developed grey hair due to a loss of differentiated melanocytes and bald patches of skin associated with a thickening of the epidermis. We found that the balance of cell proliferation to differentiation was disturbed in the skin of these mice apparently because the over-duplication of centrosomes does not permit primary cilia to form. Because the primary cilia are necessary for signalling between cells, this perturbs cell differentiation. (Coelho et al. Open Biol. 2015)
We found that mice over-expressing Plk4 also showed hyperproliferation of pancreatic islets; these also become enlarged as a result of equal expansion of α- and β-cells, which exhibit centrosome amplification. We now study the effects of Plk4 over-expression in pancreatic organoids established from these mice.
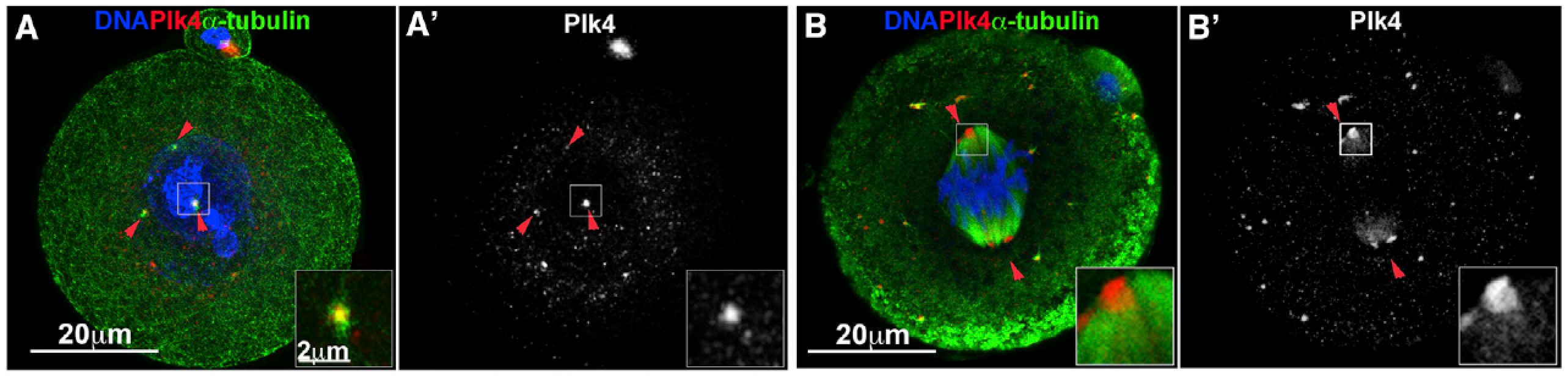
Plk4 has multiple functions at the centrosome
Plk4’s main function is to drive centriole duplication. However, clues to its other functions in nucleating microtubules have come from studies of the oocytes and early embryos of the mouse, where centrioles have yet to be assembled and the spindle becomes assembled using acentriolar microtubule organizing centres (MTOCs).
We found that, in the early mouse embryo, bipolar spindle formation requires Plk4 function in concert with its partner protein Cep152, in the absence of centrioles. However, in the oocyte, which also lacks centrioles, the MTOCs require not only Plk4 but also Aurora A to become fully active. We are currently studying this difference in spindle formation in these two acentriolar cell types. A more comprehensive understanding of spindle formation at the stages will give insights into many aspects of human fertility.
Reference: Coelho PA, Bury L, Sharif B, Riparbelli MG, Fu J, Callaini G, Glover DM, Zernicka-Goetz M. Spindle formation in the mouse embryo requires Plk4 in the absence of centrioles. Dev Cell. 2013; 27 (5): 586-97. DOI: 10.1016/j.devcel.2013.09.029.
Reference: Bury L, Coelho PA, Simeone A, Ferries S, Eyers CE, Eyers PA, Zernicka-Goetz M, Glover DM. Plk4 and Aurora A cooperate in the initiation of acentriolar spindle assembly in mammalian oocytes. J Cell Biol. 2017; 216 (11): 3571-3590. DOI: 10.1083/jcb.201606077.
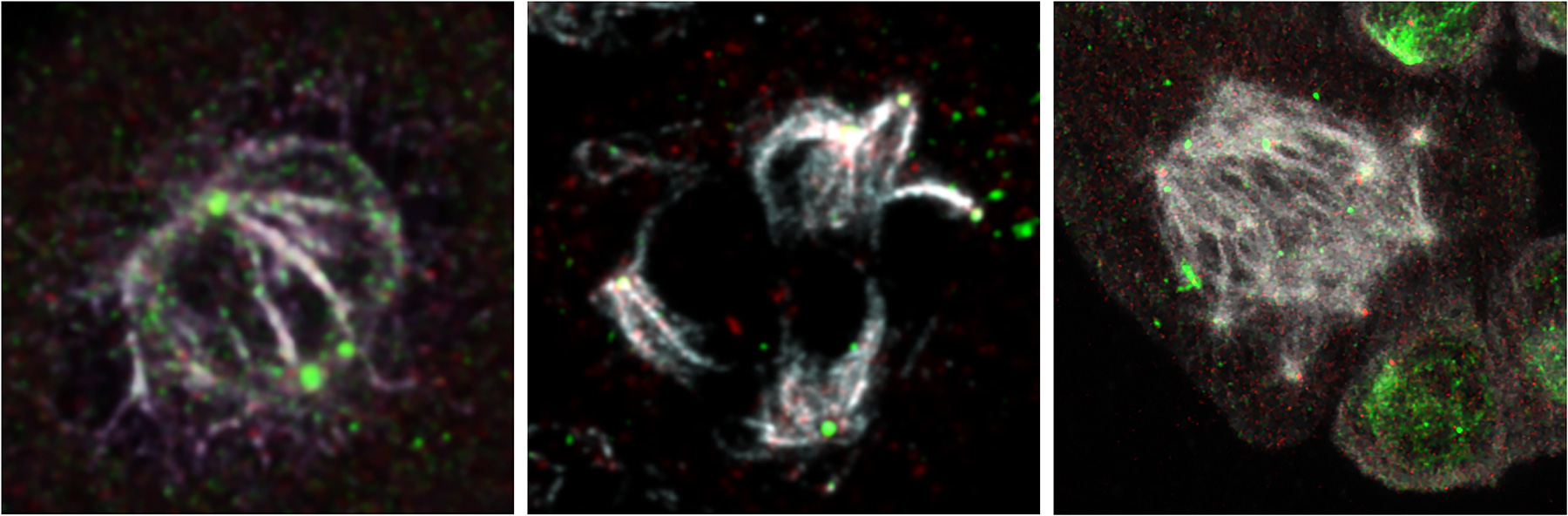
Multiple pathways controlling centrosome numbers
Although significant progress has been made in identifying functional players in centrosome duplication, we still need to improve our understanding of the centrosome’s role in tumorigenesis and inherited disease. Our team has conducted a CRISPR/Cas9 genome-wide screen in mouse embryonic stem cells and discovered new mechanisms by which cells respond to centrosome amplification. This is helping us better understand how cancer cells become permissive to supernumerary centrosomes. We are currently studying the crucial role of the Rho GTPase pathway in signalling centrosome amplification and in triggering downstream responses, including increased vesicle trafficking and autophagy, to maintain centrosome homeostasis.