SPAN
Chemistry, discharge reactions
click images to enlarge:
Dacheng Kuai, Shen Wang, Saul Perez-Beltran, Sicen Yu, Gerard A. Real, Ping Liu, and Perla B. Balbuena, “Interfacial Electrochemical Lithiation and Dissolution Mechanisms at Sulfurized Polyacrylonitrile Cathode Surface,” ACS Energy Lett., (2024), 9, 3, 810–818
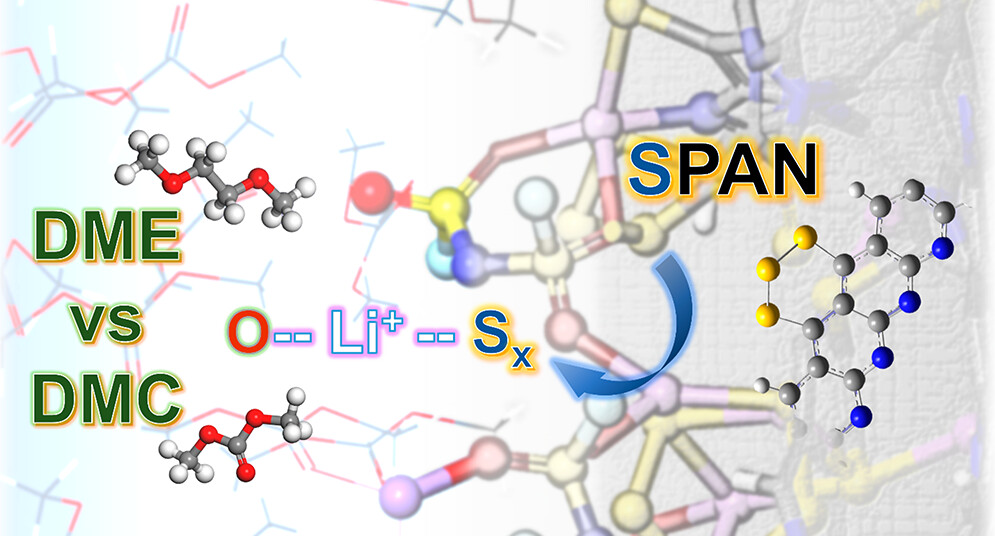
Advances in sulfurized-polyacrylonitrile (SPAN)-based cathode materials promise safer and more efficient lithium–sulfur (Li-S) battery performance. To elucidate electrolyte–cathode interfacial electrochemistry and polysulfide (PS) dissolution, we emulate discharge SPAN reactions via ab initio molecular dynamics (AIMD) simulations. Plausible structures and their lithiation profiles are cross-validated via Raman/IR spectroscopy and density functional theory (DFT). Lithium bis(fluorosulfonyl)imide (LiFSI) plays versatile roles in the Li-SPAN cell electrochemistry, primarily as the major source in forming the cathode–electrolyte interphase (CEI), further verified via X-ray photoelectron spectroscopy and AIMD. Besides being a charge carrier and CEI composer, LiFSI mediates the PS generation processes in SPAN electrochemical lithiation. Analysis of AIMD trajectories during progressive lithiation reveals that, compared to carbonates, ether solvents enable stronger solvation and chemical stabilization for both salt and SPAN structures. Differentiated CEI formation and electrochemical lithiation decomposition pathways and products are profoundly associated with the intrinsic nature of lithium bonding with oxygen and sulfur.
Saul Perez Beltran and Perla B Balbuena, “Sulfurized Polyacrylonitrile (SPAN): Changes in Mechanical Properties during Electrochemical Lithiation,” J. Phys. Chem. C, 125, 24, 13185-13194, (2021).
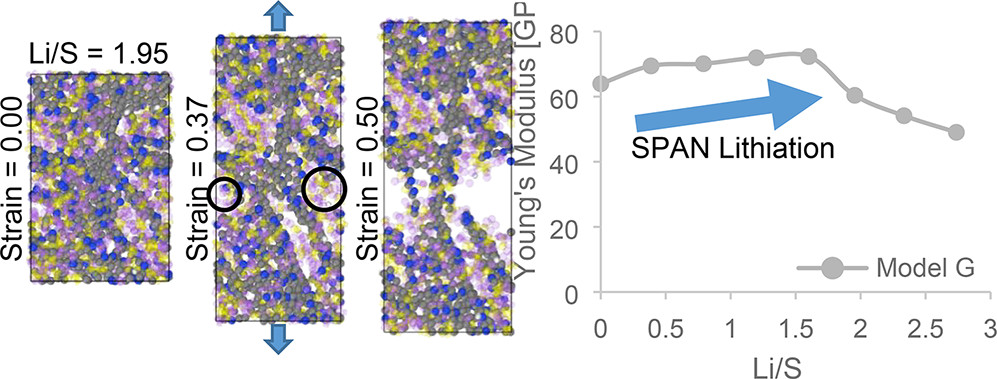
Sulfurized polyacrylonitrile (SPAN) is a promising material for stable lithium–sulfur (Li–S) batteries that can potentially satisfy the demand for high-density energy storage devices for electric vehicles (EVs). However, important physical and chemical properties of the SPAN cathode material are not yet well understood. For example, the SPAN mechanical behavior that depends on the structure and chemistry of the material generated during synthesis and the mechanical response evolution during battery discharge have been scarcely investigated. This work addresses the effects of electrochemical lithiation on the SPAN mechanical integrity via uniaxial tensile loading tests using molecular dynamics with the ReaxFF potential. We evaluate the volume expansion, Young’s modulus, yield strength, and ultimate tensile strength with increasing lithium contents. Our results show how the degree of graphitization of the carbonized skeleton impacts the SPAN ability to withstand the volume expansion-induced structural stresses upon lithiation. We describe the fracture mechanisms and find out a ductility loss with increasing lithium contents.
Saul Perez Beltran and Perla B Balbuena, “A Solid Electrolyte Interphase to Protect the Sulfurized Polyacrylonitrile (SPAN) Composite for Li-S Batteries: Computational Approach Addressing the Electrolyte/SPAN Interfacial Reactivity,” J. Mater. Chem. A, 9, 7888-7902, (2021).
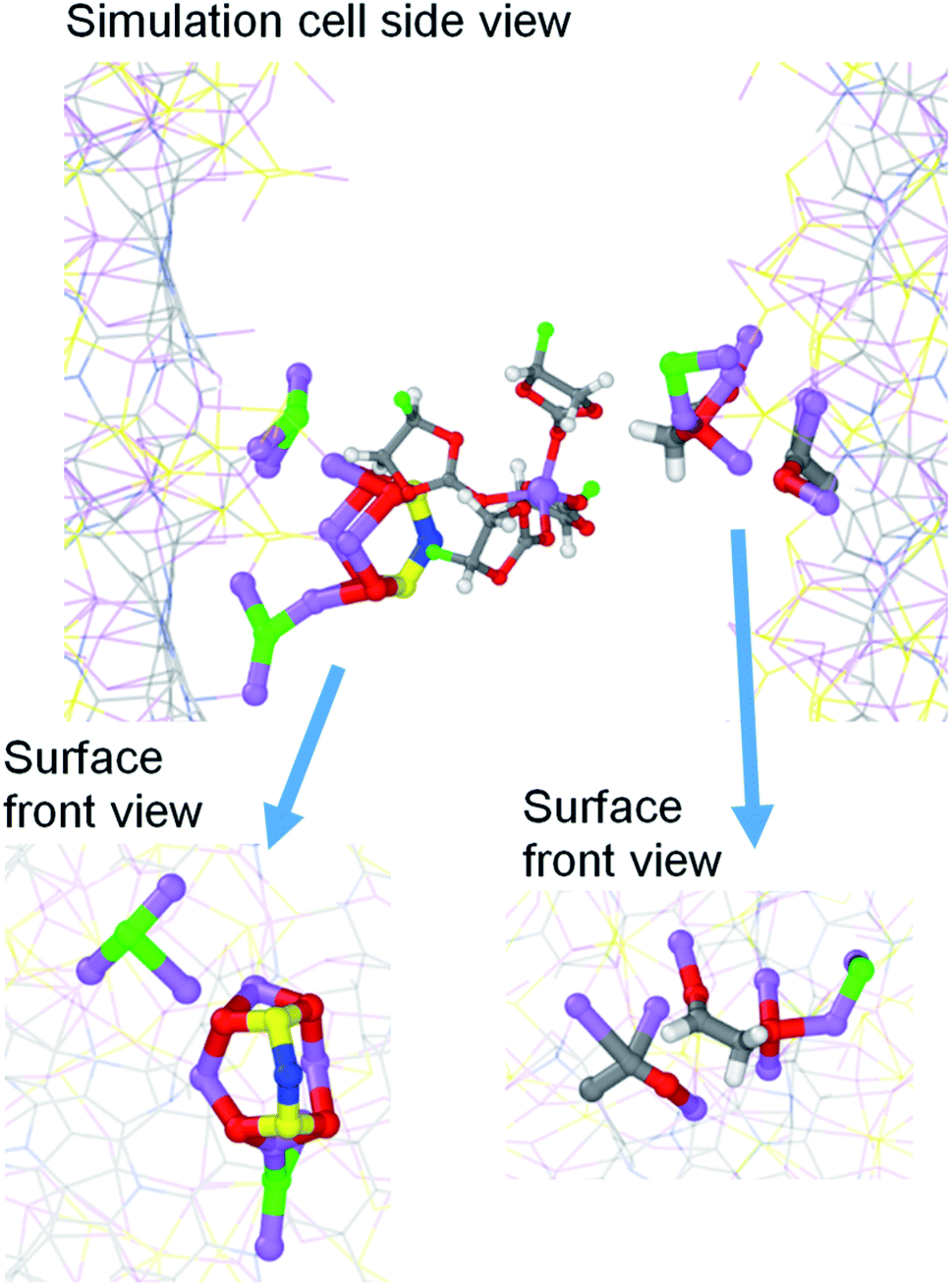
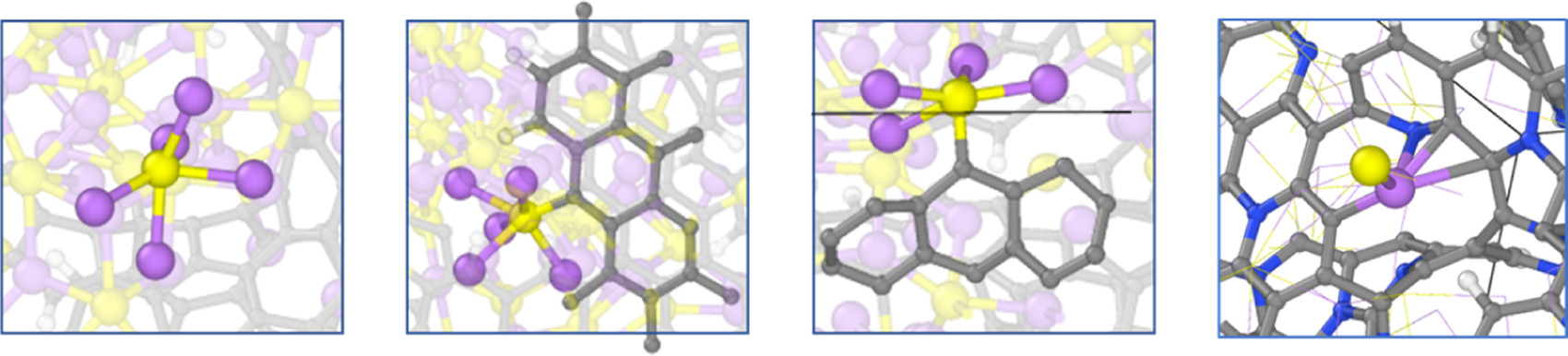
This study addresses the reactivity of multiple solvents and lithium bis(fluorosulfonyl)imide (LiFSI) at the interface with sulfurized polyacrylonitrile (SPAN) in multiple stages of lithiation via ab initio molecular dynamics simulations. Both ether 1,3-dioxolane (DOL) and dimethyl carbonate (DMC) proved stable on the lithiated SPAN surface regardless of the lithium content, meaning that neither of these species likely contributes to growing a solid electrolyte interphase (SEI) coating to protect the SPAN composite. Conversely, cyclic carbonates, ethylene carbonate (EC) and fluoroethylene carbonate (FEC) are shown to be very active. The EC reduction occurs only on a highly lithiated surface with a 3.0 Li/S molar ratio, the equivalent of the SPAN composite in an over-discharge regime with voltages close to 0.0 V vs. Li/Li+. The FEC reduction starts with a 2.0 Li/S molar ratio and above, suggesting that FEC could act as a useful additive in electrolyte formulations with EC. Both EC and FEC follow multiple reduction mechanisms to produce complex reduction products and LiF in the FEC case. We provide a mechanistic description for each detected decomposition path. The LiFSI salt also proves reactive against the lithiated SPAN surface. The FSI− defluorination is the dominant reduction path. However, the SO2NSO2F− and SO2NSO22− species proved stable against S–N cleavage. This behavior makes the LiFSI salt a potential candidate for SPAN-based Li–S batteries because it produces LiF without releasing SO2.
Saul Perez Beltran and Perla B. Balbuena, “Sulfurized Polyacrylonitrile for High-Performance Lithium-Sulfur Batteries: In-Depth Computational Approach Revealing Multiple Sulfur’s Reduction Pathways and Hidden Li+ Storage Mechanisms for Extra Discharge Capacity,” ACS Appl. Mater. & Interfaces, 13, 1, 491-502, (2021).
Like no other sulfur host material, polyacrylonitrile-derived sulfurized carbon (SPAN) promises improved electrochemical performance for lithium–sulfur batteries, based on its compatibility with carbonate solvents and ability to prevent self-discharge and shuttle effect. However, a complete understanding of the SPAN’s lithiation mechanism is still missing because its structural features vary widely with synthesis conditions, and its electrochemical performance deviates from elemental sulfur. This study continues our research on the elucidation of the SPAN’s structural characteristics and lithiation mechanisms via computational approaches. Our models reproduce most experimental data regarding carbon’s graphitization level and conjugated ordering, sulfur–carbon covalent bonding, sulfur loading, and elemental composition. Our simulations emulate the discharge voltage observed in experiments for the first discharge, which reveals that sulfur follows multiple reduction pathways based on its interaction with the carbon backbone. Sulfur reduction takes place above 1.0 V vs Li/Li+ mostly in the SPAN-like material, with no long-chain lithium polysulfide formation. Below 1.0 V vs Li/Li+, the backbone’s electrochemical activity occurs via multiple C–Li and N–Li interactions, mostly with edge carbon atoms and pyridinic nitrogen. Moreover, we identify Li+ binding sites throughout the graphitized backbone that might lead to prohibited energy costs for Li+ deintercalation, which may explain the irreversible capacity loss between the first and second discharges. This work improves understanding of lithiation mechanisms in sulfurized carbon, which is useful for rationally designing SPAN synthesis pathways tailored to increase sulfur loading and enhanced electrochemical performance.
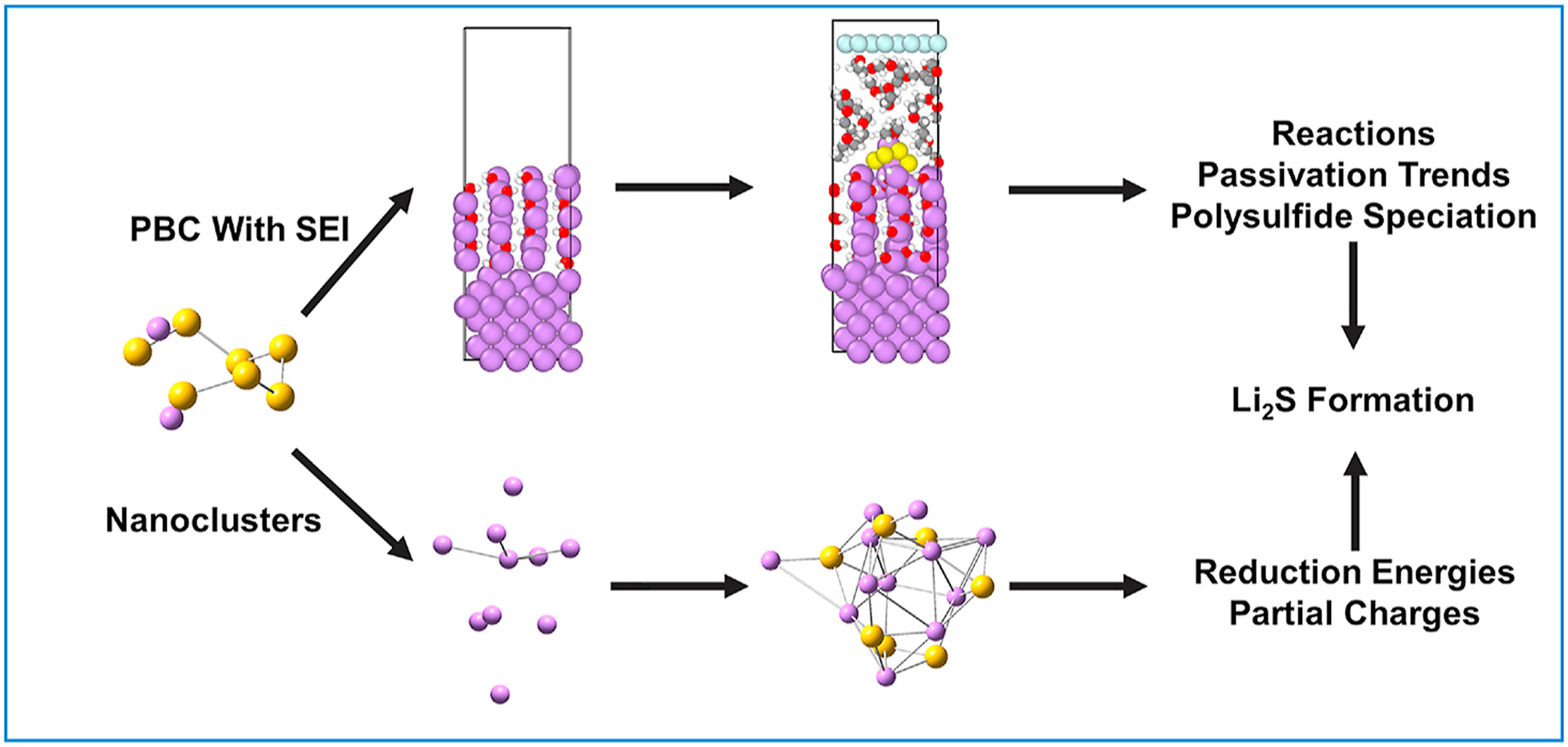
E. P. Kamphaus and P. B. Balbuena, “Polysulfide Reduction and Li2S Phase Formation in the Presence of Lithium Metal and Solid Electrolyte Interphase Layer,” J. Power Sources, 485, 229289, (2021).
Lithium sulfur battery is an attractive next generation technology that can meet many demands of modern society. Unfortunately, the lithium sulfur battery faces unique issues related to the polysulfide shuttle effect, that is due to reduction products dissolving in the electrolyte and their subsequent reduction on the lithium metal electrode. This adds further problems to the already challenging needs of understanding and engineering a solid electrolyte interphase (SEI) layer with desired properties. One of the most important SEI properties is its passivation of lithium metal which is critically important to the overall battery performance. Passivation is difficult to measure experimentally without the influence of many factors. This study reports an investigation of the reduction of the intermediate Li2S8 over lithium already passivated with Li2O, Li2CO3, LiOH, LiF and Li2S along with exploration of Li2S8 reduction over pristine lithium nanoclusters using first principles computational models. Significant formation of Li2S phase nucleation is found to stabilize the reduction products of the Li2S8. The formation of Li2S is explored in-depth with lithium nanocluster-based models determining a 2 V potential increase for the reduction of polysulfides due to the formation of Li2S. This investigation demonstrates passivation effects of important SEI components including Li2S.
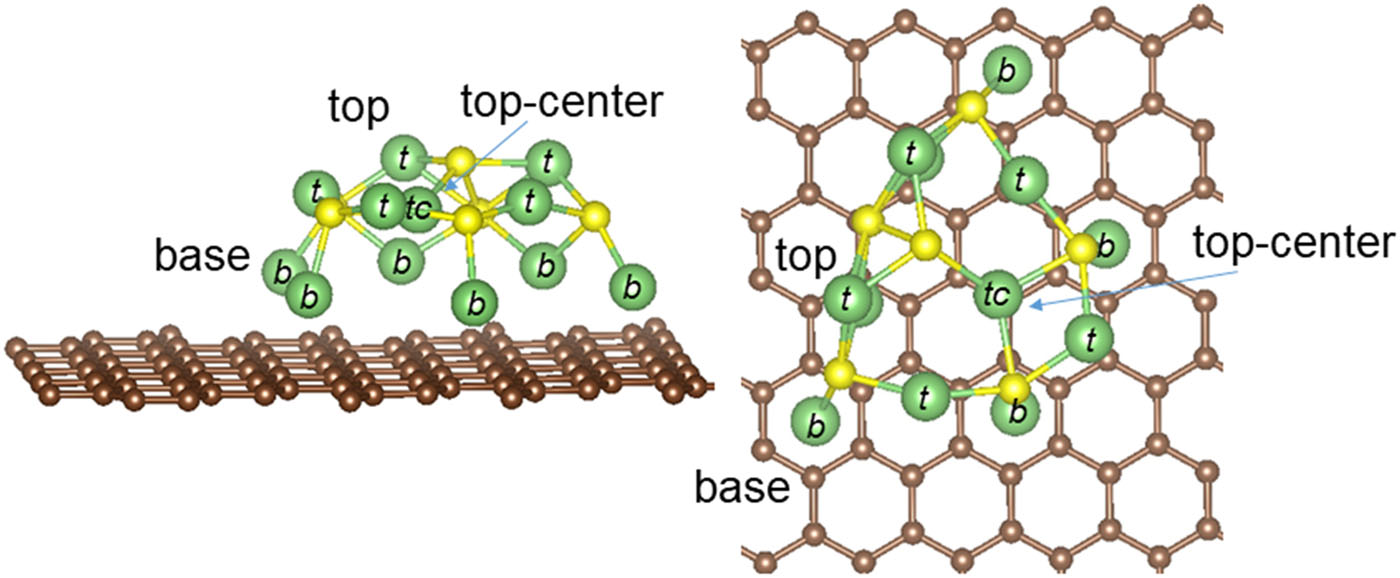
Roberto C. Longo, Luis E. Camacho-Forero, Perla B. Balbuena, “Li2S Growth on Graphene: Impact on the Electrochemical Performance of Li-S Batteries,” J. Chem. Phys., 152, 014701, (2020).
Lithium-sulfur batteries show remarkable potential for energy storage applications due to their high-specific capacity and the low cost of active materials, especially sulfur. However, whereas there is a consensus about the use of lithium metal as the negative electrode, there is not a clear and widely accepted architectural design for the positive electrode of sulfur batteries. The difficulties arise when trying to find a balance between high-surface-area architectures and practical utilization of the sulfur content. Intensive understanding of the interfacial mechanisms becomes then crucial to design optimized carbon-hosted sulfur architectures with enhanced electrochemical performance. In this work, we use density functional theory (DFT)-based first principles calculations to describe and characterize the growing mechanisms of Li2S active material on graphene, taken as an example of a nonencapsulated carbon host for the positive electrode of Li-S batteries. We first unravel the two growing mechanisms of Li2S supported nanostructures, which explain recent experimental findings on real-time monitoring of interfacial deposition of lithium sulfides during discharge, obtained by means of in situ atomic force microscopy. Then, using a combination of mathematical tools and DFT calculations, we obtain the first cycle voltage plot, explaining the three different regions observed that ultimately lead to the formation of high-order polysulfides upon charge. Finally, we show how the different Li2S supported nanostructures can be characterized in X-ray photoelectron spectroscopy measurements. Altogether, this work provides useful insights for the rational design of new carbon-hosted sulfur architectures with optimized characteristics for the positive electrode of lithium-sulfur batteries.