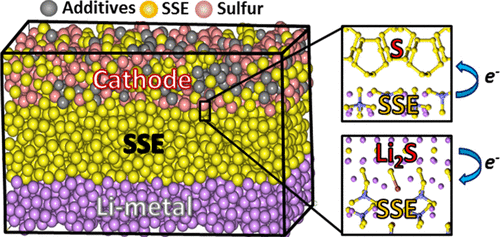
Lithium-Sulfur Batteries
Discharge reactions, polysulfide shuttle effects, charge reactions
click images to enlarge:
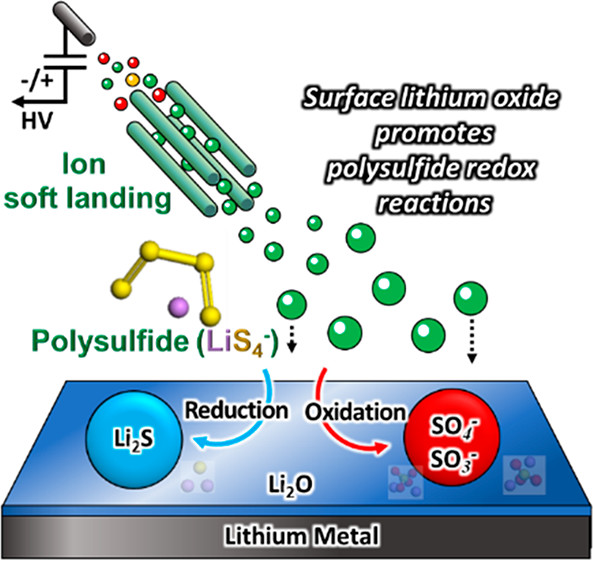
K. Hankins, V. Prabhakaran, S. Wi, V. S. V. Shutthanandan, G. E. Johnson, S. Roy, H. Wang, Y. Shao, S. Thevuthasan, P. B. Balbuena, K. T. Mueller, and V. Murugesan “Role of Polysulfide Anions in Solid-Electrolyte Interphase Formation at the Lithium Metal Surface in Li–S Batteries,”, J. Phys. Chem. Lett., 12, 38, 9360-9367, (2021).
Lithium (Li) metal battery technology, renowned for its high energy density, faces practical challenges, particularly concerning large volume change and cell swelling. Despite the profound impact of external pressure on cell performance, there is a notable gap in research regarding the interplay between external pressure and the electroplating behaviours of Li+ in large-format pouch cells. Here we delve into the impact of externally applied pressure on electroplating and stripping of Li in 350 Wh kg−1 pouch cells. Employing a hybrid design, we monitor and quantify self-generated pressures, correlating them with observed charge–discharge processes. A two-stage cycling process is proposed, revealing controlled pouch cell swelling of less than 10%, comparable to state-of-the-art Li-ion batteries. The pressure distribution across the cell surface unveils a complex Li+ detour behaviour during electroplating, highlighting the need for innovative strategies to address uneven Li plating and enhance Li metal battery technology.
K. Hankins, V. Prabhakaran, S. Wi, V. S. V. Shutthanandan, G. E. Johnson, S. Roy, H. Wang, Y. Shao, S. Thevuthasan, P. B. Balbuena, K. T. Mueller, and V. Murugesan “Role of Polysulfide Anions in Solid-Electrolyte Interphase Formation at the Lithium Metal Surface in Li–S Batteries,”, J. Phys. Chem. Lett., 12, 38, 9360-9367, (2021).
Lithium (Li) metal battery technology, renowned for its high energy density, faces practical challenges, particularly concerning large volume change and cell swelling. Despite the profound impact of external pressure on cell performance, there is a notable gap in research regarding the interplay between external pressure and the electroplating behaviours of Li+ in large-format pouch cells. Here we delve into the impact of externally applied pressure on electroplating and stripping of Li in 350 Wh kg−1 pouch cells. Employing a hybrid design, we monitor and quantify self-generated pressures, correlating them with observed charge–discharge processes. A two-stage cycling process is proposed, revealing controlled pouch cell swelling of less than 10%, comparable to state-of-the-art Li-ion batteries. The pressure distribution across the cell surface unveils a complex Li+ detour behaviour during electroplating, highlighting the need for innovative strategies to address uneven Li plating and enhance Li metal battery technology.
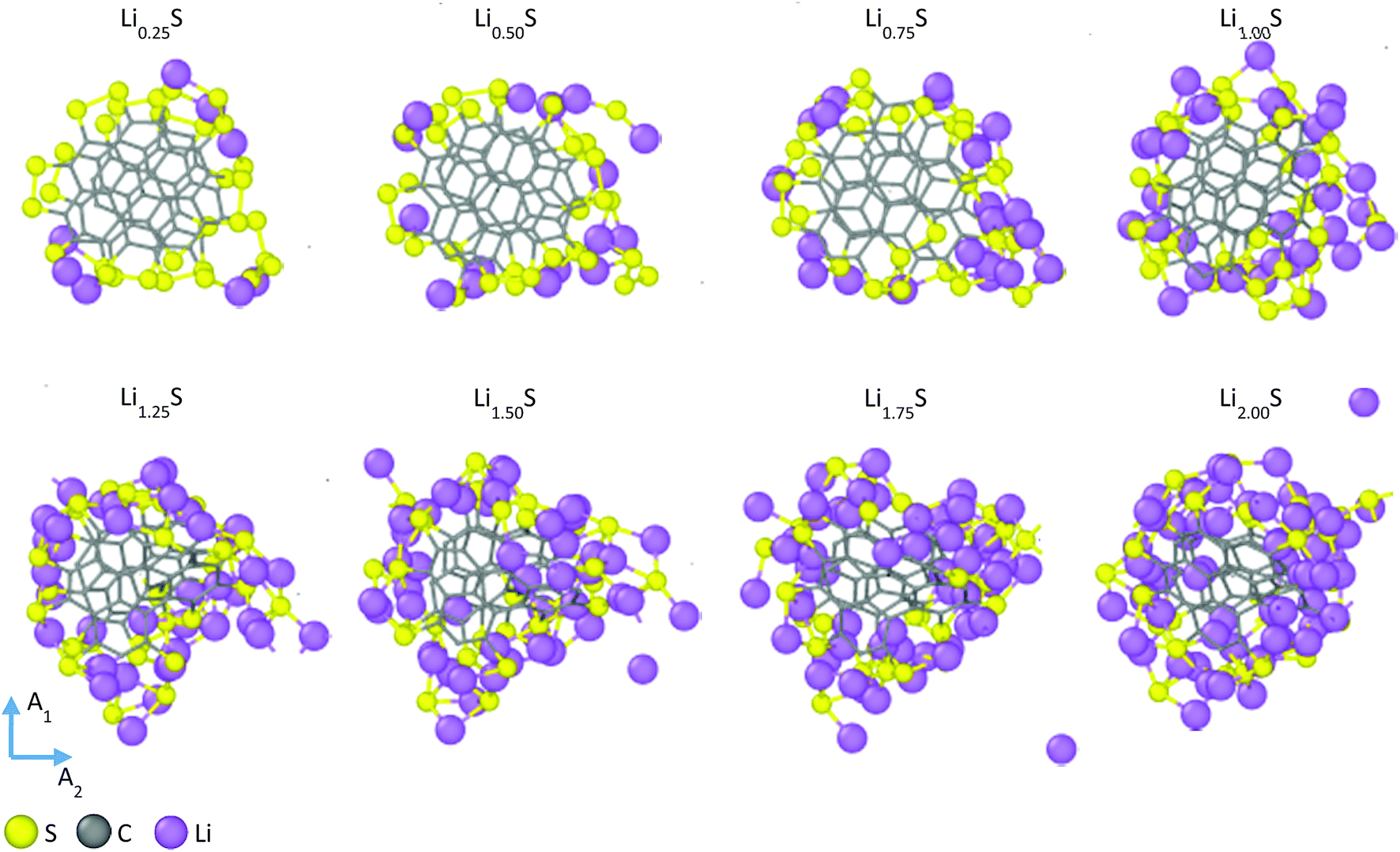
Saul Perez Beltran and Perla B. Balbuena, “First-Principles Explorations of the Electrochemical Lithiation Dynamics of a Multilayer Graphene Nanosheet-based Sulfur-Carbon Composite,” J. Mater. Chem. A, 6, 18084-18094, (2018).
Graphitized-polymer-based sulfur cathodes have emerged as alternative cathode materials that are able to overcome many of the technical challenges that currently hinder lithium–sulfur (Li–S) batteries from their use in long-term high-energy applications. However, even though graphitized-polymer-based sulfur cathodes can be synthesized through a facile thermal procedure, and there is evidence of sulfur–carbon chemical bonding, little is known about the electrochemical lithiation processes in these structures. So far, no mechanistic study has followed the dynamics of the discharging process, and no theoretical study has addressed why certain Li–S batteries based on graphitized-polymer-based sulfur cathodes have shown an initial discharge capacity higher than the theoretical discharge capacity of sulfur (1672 mA h g−1). Here we use ab initio molecular dynamics simulations as a tool to follow the electrochemical lithiation processes in a multilayer graphene nanosheet-based sulfur–carbon composite model embedded in a liquid 1,3-dioxolane solvent phase. Our model reproduces the main structural motifs associated with graphitized-polymer-based sulfur cathodes: chemical sulfur–carbon bonding and π–π stacking for the carbon backbone. Our results indicate that sulfur–carbon bonding prevents sulfur from retaining its stable cyclic structure before lithiation yielding a distribution of linear sulfur chains tightly bonded to the carbon backbone. During lithiation, we observe a competing effect for the incoming electrons between the sulfur atoms and the carbon backbone. This effect induces the partial reduction of the carbon backbone leading to accumulation of additional lithium around it, favoring the lithiation process beyond a 2 : 1 Li : S molar ratio. As a consequence, it also limits the formation of extended Li2S-like structures as the final reduction products. Finally, comparison of the calculated voltage discharge profile of our model shows close agreement with reported experimental voltage profiles based on sulfur/polyacrylonitrile (SPAN) cathodes, with the proposed mechanisms providing a plausible structural explanation for the additional discharge capacity of SPAN-based cathodes compared to the theoretical discharge capacity of sulfur.